

The pharmaceutical sector increasingly uses digital technologies to support all aspects of the product lifecycle, from its conception and design to validation, manufacturing, servicing and disposal. However, with new technological advances and modernization of labs, old technologies, particularly software, become obsolete very quickly. In particular, software becomes unusable and data inaccessible.
Software is needed for as long as the data is retained. This has crucial consequences for our access to electronic data that has been collected and analysed by computerized systems. Without software, data-files cannot be opened and digital data cannot be read, processed, and interpreted.
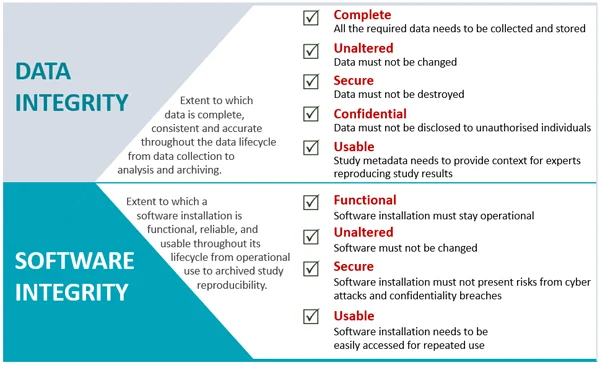
GxP regulations require that the data must be retained and that data integrity must be ensured throughout the product lifecycle. In particular, regulatory guidance from OECD state that raw data collected from instruments during discovery and pre-clinical research have to be archived in their original form and used for reconstruction of studies during regulatory audits. Software obsolescence makes meeting the GxP regulatory requirements a challenge.
Number 15
Establishment and Control of Archives that Operate in Compliance with the Principles of GLP Introduction
The maintenance of the raw data associated with a specific study and the specimens generated from that study are the only means that can be used to reconstruct the study, enabling the information produced in the final report to be verified and the compliance with GLP of a specific study to be confirmed.
Number 17
Application of GLP Principles to Computerised Systems
3.2 Data and storage data
When a system or software is updated, it must be possible to read data stored by the previous version or other methods must be available to read the old data...
Software should be retained in the archive if necessary to read or reconstruct data.
Fortunately, old software and operating systems can now be installed in virtual machines. While physical computing devices have their own operating systems, the virtual machines are ‘software computers’ which can run on other host computers. Thus, using virtual machines eliminates risk of hardware failure. Virtual machines can be accessed remotely from modern devices.
HMRC and OECD has recognized the potential and importance of virtual machines in managing legacy software that is needed to read the archived study data.
Intact Digital uses state-of-the-art virtualization and remote access technologies to enable use of legacy software.
6.17.1 Archive
When legacy systems can no longer be supported, considerations should be given to maintaining the software for data accessibility purposes as long as reasonably practicable. This may be achieved by maintaining software in a virtual environment.
INTACT Software Library provides a platform and services for installing, validating, and hosting software installations needed for long-term readability and use of data.
We host your legacy software in Intact Digital data centers and enable its use through secure virtual desktops. That way, you have validated and carefully maintained readers of your archived data, enabling reliable reconstruction of past studies.

INTACT Software Library complements your electronic data archives and supports archivists and scientists in meeting the requirements of GxP regulatory audits.
A study reconstruction requires data from the archive to be transferred securely to the Software Library environment for a temporary use with a specified and validated software installation.
The original archived data stays unchanged and unaffected by the study reconstruction process. The used data copy is removed from the Software Library when the task is completed.

An authorized user can use a modern and secure device to login into the Software Library through a standard Internet Browser. Virtual Desktops, with pre-installed software, appear as tab windows. You can access multiple virtual machines through different browser tabs at the same time.
Data transfer is enabled through Transfer Desktop, specially configured virtual desktop to enable secure transport of a data copy into the appropriate virtual desktop for processing. Once the task of reconstructing the study is completed, the data copy is deleted and the virtual machine is closed.
Your organization and individual research teams can have their own INTACT Software Library accounts to manage software important for long-term data use.
INTACT Software Library set-up procedure is standardized and informed by requirements for regulatory compliance. It involves

Each INTACT Software Library comes with three components and flexible service and maintenance agreements to meet your specific needs.






GxP regulations require reproducibility of studies from raw data. It needs to stay readable and acccessible.
A copy of data from the eArchive is safely transferred for use with virtualized software installations.
Once the task is completed the data copy from the Software Library is removed.
1. Software is installed within securely managed virtual machines.
2. Virtual desktops provide secure remote access to software and data.
3. Software installation is validated and data analyses is reliably reproduced.
4. Virtual environments and software installations are monitored and maintained.


Digital data requires software to be accessed and used. Yet, over time, every software comes to the end-of-support, when the software producer stops updating it, or to the end-of-life, when the whole product is discontinued.
Once lab instruments and software are decommissioned, the integrity of archived data is at risk – there is no software to ensure its long-term readability and, therefore, no means to stay compliant with regulations on data integrity.
INTACT Software Library takes care of the software in the archiving phase, ensuring that the software is always ready to be used.
INTACT Software Library is aligned with the MHRA recommendations and ensures your data is “ALCOA+” compliant so you don't have to worry about these concerns.

INTACT Software Library offers a systematic and principled approach to long-term use of software by removing the hardware failure risks and the operating system security risks.
Each organization and lab can have its own Software Library with virtualized installations of legacy software. Software installations are hosted in a private data center where security of the non-secure software can be carefully managed. Since installed in virtual machines, the software is also safe from hardware failures.
Data that needs to be used with the software is carefully transferred into the Software Library environment. A copy of selected archived data is exported for use within the Software Library through a secure transfer desktop. Once transferred, the data is used to complete the task and then deleted. No archived data is altered or affected in any way.
Only copies of the data are used and processed for study reconstruction.
INTACT Software Library design and procedures enable organizations to adhere to their internal policies on software and data management. The software installations are carefully created since any changes to the software or the environment within which the software operates may introduce inconsistencies and affect reproducibility of past studies. Even newer, improved and upgraded software versions may not be used if one cannot guarantee that there are no discrepancies in the way data is processed and visualized.
Study reproducibility requires software installations that are as close to the original as possible. That can be achieved by incorporating relevant aspects of the original CSV process, as it has been demonstrated through a case study of Analyst 1.4.2 software by SCIEX, originally installed in a bioanalysis lab in 2006.
We offer flexible subscription plans to meet your long-term needs.
Your Software Library expands over time as software technologies become obsolete.
Easily add new software, enrol new users, and specify access privileges.